
LinkCor® Bioengineered Corneal Implant for treating Advanced Keratoconus
LinkCor® is a biocompatible corneal implant for managing corneal blindness and impairment, primarily used for treating patients suffering from Advanced Keratoconus. Keratoconus is a progressive eye disorder characterized by bulging, thinning, and scarring of the cornea.
The implant is 100% natural and made of collagen fibers, which is the same material the human cornea is mainly comprised of. It is designed to mimic the human cornea while offering superior optical properties and enhanced oxygen and nutrient permeabilities than the human cornea.
LinkCor® is manufactured under aseptic and GMP-compliant conditions and supplied in sterile form. It is made from highly purified collagen and is free from human and animal cells, and free from any impurities or biological microorganisms, which may cause rejection and inflammation. That’s why LinkCor® is highly biocompatible and well-accepted by the patient’s eye after implantation. The novel material is fully crosslinked with fixed swelling properties, making it less sensitive to environmental factors that can cause extreme corneal swelling and opacity resulting in low vision or blindness.
LinkCor® comes in the shape of an implantable contact lens at different thicknesses for treating keratoconus. As reported in our Nature Biotechnology paper (https://doi.org/10.1038/s41587-022-01408-w), safety and efficacy measures at the two-year post-operative clinical trial period of LinkCor® implants in 20 patients confirmed that:
- All patients regained their sight upon LinkCor®
- Corneal transparency was maintained post-operatively in all subjects, with no reported incidents of corneal haze (compared to 35% of patients suffering haze with synthetic corneal implants).
- No rejection, inflammation, vascularization, scarring or other adverse events occurred in any subject. This was mainly due to using sterile, ultra-pure, medical-grade collagen as a starting material and the advanced GMP manufacturing processes. Furthermore, the device is comprised of pure stabilised collagen and water without any cells, biological components, or chemicals. Therefore, the human body’s immune system does not sense the implant as a foreign substance, therefore, no rejection or inflammation takes place.
LinkCor® requires a minimally invasive, relatively affordable implantation procedure with a quick recovery period (<4 weeks accompanied by a light drug regimen).
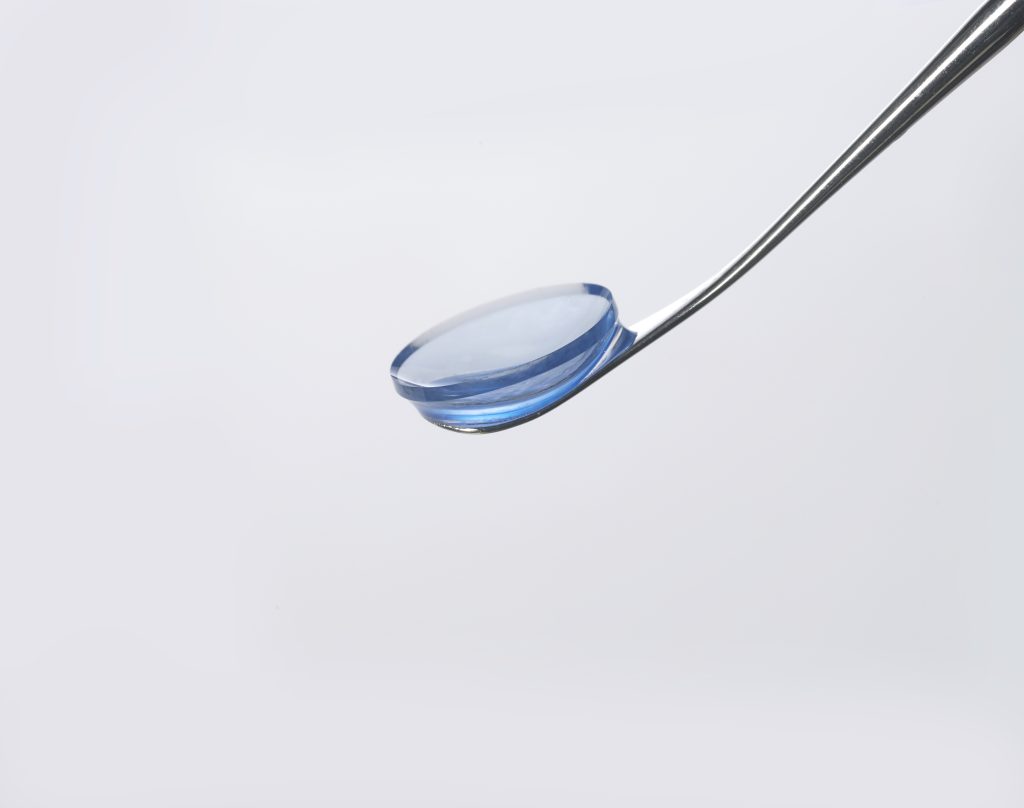
CorVision® Bioengineered Corneal Microlens for managing presbyopia.
CorVision® is a natural corneal microlens manufactured from the same material that the human cornea is made of, e.g. collagen. The microlens integrates in the corneal environment in long-term avoiding biocompatibility, haze, and interface issues associated with other microlenses.
It eliminates the need for reading spectacles, contact lenses, or more invasive surgeries such as LASIK.
Our CorVision® bioengineered corneal microlens holds promise for such applications addressing the biocompatibility and the irreversibility issues of the existing techniques while improving uncorrected near vision.
CorVision® in many respects is superior to other inlays as it is biocompatible (vs. KAMRA and Raindrop), reproducible with smooth surface and edges, can be customized and easily mass-produced. CorVision microlens is also placed in a corneal pocket, a major advantage of the pocket technique, compared to the flap technique, is the salvation of more peripheral corneal nerves. This defends against the diminished corneal sensation associated with flap creation, which in turn allows for a reduced incidence of dry eyes and a potential faster visual recovery.
Presbyopia: what it is and how it affects us:Presbyopia is an age-related progressive loss of near vision caused by reduced crystalline lens accommodation power. In a normal eye, when focusing on a near object, the lens bulges (more convex) to bring the image into sharp focus on the retina. However, in presbyopia, this capability of the lens is lost to some extent resulting in the image to focus behind the retina causing blurring of vision at near, especially in reduced illumination.
It is estimated that presbyopia affects an estimated 1.1 billion people aged 35 years and older. The onset age of presbyopia is around 40 years, and the prevalence is 100% by age 50 meaning that Presbyopia affects all people over the age of 50. Patients with presbyopia are usually at high risk of age-related diseases.
Current treatment/management options for Presbyopia:
Non-surgical and surgical methods are used to improve near vision e.g., for reading and clear near vision. Non-surgical methods of presbyopia correction include eyeglasses and contact lens options, while the surgical correction includes laser surgeries, synthetic corneal inlays, and intraocular lenses which remain as significant challenges for refractive surgeons.
Overall, there have been significant advances in surgical management of presbyopia over the last 30 years resulting in relatively acceptable outcomes, but each method has its own advantages and disadvantages. For instance, even though excimer laser based refractive surgeries are relatively safe and effective, their irreversible nature due to the removal of corneal tissue makes them less attractive in the long term. As for the lens-based procedures, the intraocular procedures are in general often considered too invasive for presbyopia correction. Considering these shortcomings, development of new reversible technologies that are based on natural materials is of high importance.

LinkCell™ Bioengineered Substrate/Carrier for human or animal cells and stem cells
LinkCell™ has unique characteristics:
- It is 100% natural and made of collagen fibers, which is the main building block material of the human body. It is designed to mimic the human tissues such as the cornea.
- Due to its high transparency and biocompatibility, LinkCell™ is a great substrate for cells growth and visualisation.
- Though ultra-thin, LinkCell™ is mechanically robust and resistant to biodegradation.
- The novel material is fully crosslinked with fixed swelling properties, making it less sensitive to environmental factors while offering superior oxygen and nutrient permeabilities.
LinkCell™ can also be used as a substrate material for research and development towards enhanced growth and differentiation of all cell types.
LinkCell™ can also be used as an alternative tissue-mimetic to animal testing in the safety assessments for chemicals and drug formulations, cosmetics, personal care, and consumer products.
LinkCell™comes in flat sheet form at different thicknesses (20 µm to 200 µm) and diameters (2 mm to 200 mm).
LinkCell™ superior properties and performance are already published in peer-reviewed scientific journals:
